Adrenal Insufficiency United is excited to share this update from Adrenas on their gene therapy trial for Congenital Adrenal Hyperplasia. We are thankful for this dedicated group of individuals who care deeply for our community.

AIU has provided this addition information to aid in your understanding of the graph seen in the letter below.
- Data from 2 Participants dosed at level 3
- Pt = Participant
- Pt 6 12-week data pending
January 8, 2024
Dear CAH Community Members,
Adrenas Therapeutics, a BridgeBio company, appreciates your ongoing interest in our investigational gene therapy for adults with classic Congenital Adrenal Hyperplasia (CAH) due to 21-hydroxalyse deficiency. We are pleased to share an update on the phase 1/2 ADventure trial.
– Participants and Dose Levels: A total of seven participants have been dosed with Adrenas’ investigational gene therapy, BBP-631: two participants at each of Dose Levels 1, 2, and 3. An additional Dose Level 4 has been added in response to encouraging emerging data. One participant has received Dose Level 4, with a second participant planned for early 2024.
– Observed Safety: The investigational gene therapy has been well tolerated by all participants. To date there has been a single serious adverse event at Dose Level 1 related to redness at the infusion site, which fully resolved and which was deemed by the treating physician to be unrelated to the gene therapy. No further skin reactions were observed in Dose Levels 2 through 4. Of course, more time and data from more participants are still needed to characterize the safety profile of BBP-631. Based on the detailed evaluation of each participant’s safety data, as well the overall safety profile to date of BBP-631, an independent Data Safety Monitoring Commitee (DSMC) approved each dose escalation through Dose Level 4. All participant data will continue to be evaluated at regular intervals by the DSMC.
‒ Potential Efficacy: Early data show robust changes in a direct precursor to the production of cortisol, 11-deoxycortisol, in those participants dosed at higher doses. The increase in 11-deoxycortisol reflects 21-hydroxylase activity and is translating into an early, steady increase in cortisol production. While more data are needed to explore the magnitude of cortisol production at higher doses of BBP-631 and also to fully characterize the durability of this effect, the current data represent the first demonstration of an investigational approach allowing people living with CAH to increase their own (endogenous) production of cortisol.
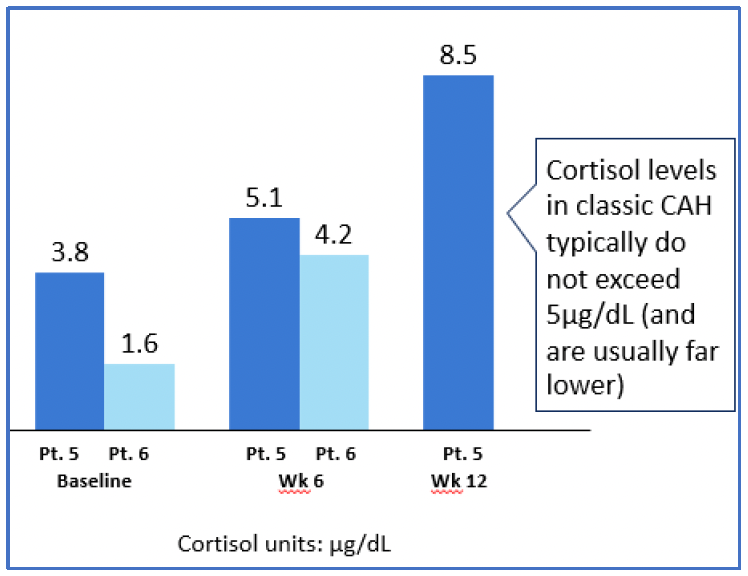
Adrenas’ goal for the ADventure trial is to confirm the safety and potential efficacy of Adrenas’ investigational gene therapy on adrenal-related hormones at a variety of dose levels, aiming to find an optimal dose level before advancing the program. While we are encouraged by this progress, Adrenas will continue to collect and closely review all data on the potential impact of BBP-631 in people living with CAH. We will continue to update the community with our evolving understanding later in 2024.
Adrenas acknowledges the tremendous contributions of those participating in the ADventure trial, as well as the patient advocacy groups, clinical research sites and investigators, and the broader CAH community. We are grateful to all of you for your ongoing collaboration and support.
Sincerely,
The Adrenas Therapeutics Team
For information visit https://cahgenetherapy.com and https://clinicaltrials.gov/study/NCT04783181
